
DNA Tests for Cattle
• traits • coat color • defects •
This is a directory of the most common (most are DNA testable) heritable, genetic traits and conditions found in North American breeds of cattle. Genotyping cattle is a fast-moving science--while we try to keep this page current and complete, we welcome readers to email homestead.cattle.assn@gmail.com if anything is found missing or outdated.
|
Genetic Traits |
||||
| miscellaneous DNA testable traits | •*• | notes ~ origin ~ breed(s) |
||
| Beta Casein (milk protein mutation: A1, wild type: A2) | A1/A2 | A2 milk is associated w/ health benefits |
||
| Beta-Lactoglobulin (milk yield) | BLG | associated w/ milk yield |
||
| Breed Base Representation | BBR | indicates breed composition or purity |
||
| Calpain 316, 530 & 4751 alleles | Calpain | associated w/ meat tenderness |
||
| Fertility-Associated Antigen | FAA | higher fertility in bulls |
||
| Fescue Tolerance T-Snip™ test | test | tolerance or susceptibility to fescue toxicosis |
||
| Hair shed | HS | increased heat tolerance |
||
| Kappa-Casein (milk non-whey protein) | KCN | associated w/ increased cheese yield |
||
| Leptin (variants in Leptin hormone gene) | Leptin | economically favorable traits in beef cattle |
||
| Mad Cow Disease (see more: infectious diseases) | BSE | genetic resistance vs susceptibility |
||
| Myostatin mutations; Double Muscling Syndrome | DM | 9 testable variants |
||
| Parentage Verification | PV | or to establish individual genetic record |
||
| Polled vs horned; Celtic variant | Pc/h | beef breeds |
||
| Polled vs horned; Friesian variant | Pf /h | dairy breeds |
||
| Slick Hair gene | S | increased heat tolerance |
||
Coat Color Genetics |
||||
| DNA testable coat color genetics in cattle | •*• | notes ~ origin ~ breed(s) |
||
| Agouti patterned blackish | Abp |
Brahman, dairy breeds |
||
| Agouti white-bellied | Aw |
Brown Swiss |
||
| Agouti fawn | Af |
Brahman, Jersey |
||
| Agouti brindle | many |
|||
| Albinism (complete absence of pigment) | any breed |
|||
| Coat Color in Cattle (see extension) | MC1R black / red |
|||
| Dilution Dexter Dun (Brown Dilution) | Irish Dexter |
|||
| Dilution Dun (variants in the PMEL17 gene (aka SILV) | many | |||
| Dilution Heterochromia irides “White Eye” | commercial Angus (1980s) |
|||
| Dilution Oculocutaneous Hypopigmentation | Angus, Sir WMS Warrant (1978) |
|||
| Extension Black [MC1R extension gene] | ED |
most breeds |
||
| Extension Wild-Type red [MC1R extension gene] | E+ |
most breeds |
||
| Extension recessive or true red [MC1R extension gene] | e |
most breeds |
||
| Extension Telstar red [affects MC1R extension gene] | EBR |
Holstein |
||
| Red Charlie [MC1R extension gene] | • |
Simmental |
||
| Dominant Red [overrides MC1R extension gene] | AD+ |
Holstein |
||
| Spotting / Markings in Cattle | most breeds; recessive to solid |
|||
Genetic Conditions, Diseases, Disorders NOT ALL, but most of these mutations are breed-specific, autosomal recessive mutations; in which case... carriers are normal and healthy; an affected calf (homozygous) inherits 2 copies; a copy from each parent = genetic disease |
||||
| DNA Testable Conditions or Diseases | •*• | breed(s) ~ origin ~ notes |
||
| Alopecia | AL | Normande: Insoumis and Honess |
||
| Arachnomelia Syndrome-BS "Spiderleg" | AS | Brown Swiss |
||
| Arachnomelia Syndrome-SM "Spiderleg" | AS | Simmental (Semper daughter) |
||
| Arthrogryposis Multiplex (Curly Calf Syndrome) | AM | Angus; GAR Precision 1680 |
||
| Bovine Congenital Erythropoietic Protoporphyria | BCEPP | Limousin, Blonde d'Aquitaine |
||
| Bovine Leukocyte Adhesion Deficiency | BLAD | Holstein; Osborndale Ivanhoe (1952) |
||
| Brachyspina | By | Holstein |
||
| Blindness in Normande Cattle | BL | Normande |
||
| Cerebellar hypoplasia and abiotrophy | CHCA | Hereford |
||
| Cholesterol Deficiency Haplotype | HCD | Holstein; Maughlin Storm, Canada |
||
| Chediak-Higashi Syndrome | CHS | Wagyu |
||
| Claudin 16 Deficiency | CL16 | Wagyu | ||
| Citrullinemia | Cit | Holstein; Linmack Kriss King |
||
| Complex Vertebral Malformation | CVM | Holstein; Carlin-M Ivanhoe Bell, Milk'g Short |
||
| Congenital Erythropoietic Porphyria (see Protoporphyria) | CEP |
Holstein, Milking Shorthorn, Ayrshire |
||
| Congenital myoclonus (neurologic disease) | CM | Polled Hereford, Australia |
||
| Congestive Heart Failure (bovine) | BCHF | beef breeds |
||
| Contractural Arachnodactyl (Fawn Calf Syndrome) | CA | Angus, Premier Independence K N |
||
| Crooked Tail Syndrome | CTS | Belgian Blue |
||
| Crop Ear (cosmetic) | CE | Highland |
||
| Dairy Fertility Haplotypes | HH | Dairy breeds |
||
| Dairy Health Haplotypes | DH | Holstein (2019) |
||
| Deficiency of uridine monphosphate synthase | DUMPS | Holstein |
||
| Delayed Blindness | DB | Hereford (search carriers) |
||
| Developmental Duplication (Polydactylism) | DD | Angus B/R New Design 036 (1990) |
||
| Digital Subluxation | DSc | Chianina |
||
| Digital Subluxation | DSh | Shorthorn |
||
| Dwarfism, Bulldog / Chondrodysplasia | BD1 | Dexter |
||
| Dwarfism, Bulldog / Chondrodysplasia | BD2 | Dexter; Meadowpark Charles, New Zealand |
||
| Dwarfism, Bulldog / Chondrodysplasia (CHO) | BDz | Miniature Zebu |
||
| Dwarfism, Bulldog / Chondrodysplasia (CHO) | BD3? | mixed minis United States |
||
| Dwarfism, Bulldog / Chondrodysplasia (CHO) | BD•n | Nelore, Brazil |
||
| Dwarfism, Bulldog / Chondrodysplasia (CHO) | BD•h | Holstein; Energy P, polled USA bull |
||
| Dwarfism, Long-headed (proportionate) | D2 | Angus; High Valley 7D7 of 4G9 (1997) |
||
| Dwarfism, Snorter (probably eradicated) | • | Hereford; Kansas Lad > St. Loius Lad (1899)
|
||
| Factor XI Coagulation Deficiency | FXI | Holstein, Canada |
||
| Factor XI Blood Coagulation Deficiency | FXI | Wagyu |
||
| Factor XIII Blood Coagulation Deficiency | FXI | Wagyu |
||
| Fishy Off-Flavor Milk (Trimethylaminuria) | FMO3 |
Ayrshire, Swedish Red & Wht |
||
| Freemartin (DNA check for fertility in female) | XY | sterile heifer born twin to a bull |
||
| High Altitude Pulmonary Hypertenstion | HAPH | several mutations cause Brisket Disease |
||
| Hypotrichosis (patchy hair or baldness) | HY |
several mutations; several breeds |
||
| Idiopathic Epilepsy | IE | Hereford |
||
| Internal Hydrocephalus | IH | Charolais |
||
| Jersey neuropathy with splayed forelimbs | JNS | Jersey |
||
| Limber Legs | LL | Jersey |
||
| Mandibulofacial Dysostosis | MD |
Polled Hereford (SHF Wonder M326 W18) |
||
| Mannosidosis: Alpha (a) Mannosidosis | MA | Red Angus, Angus, Murray Grey |
||
| Mannosidosis: Alpha (a) Mannosidosis | MA |
Galloway |
||
| Mannosidosis: Beta (ß) Mannosidosis | MB | Salers (DNA test in 1999, defect about eliminated) |
||
| Maple Syrup Urine Disease | MSUD | all Herefords |
||
| Mulefoot (Syndactyly) | LRP4 | several variants; beef & dairy breeds |
||
| Mulefoot (Syndactyly) | LRP4 | several variants; beef & dairy breeds |
||
| Multifocal symmetrical necrotising encephalomyelopathy | Limousin, Angus, Simmental |
|||
| Neuronal ceroid-lipofuscinosis | NCL | Beefmaster |
||
| Neuronal ceroid-lipofuscinosis | NCL |
Devon, Australia |
||
| Neuropathic Hydrocephalus | NH | Angus, GAR Precision 1680 |
||
| Osteopetrosis (Marble Bone) | OS | several variants; beef & dairy breeds |
||
| Osteopetrosis (Marble Bone) | OS | several variants; beef & dairy breeds |
||
| Parrot Mouth (Brachygnathism) | • | Simmental |
||
| Paunch Calf Syndrome | PCS |
Romagnola |
||
| Progressive Ataxia | PCS |
Charolais |
||
| Protoporphyria | Proto | Limousin (about eradicated) |
||
| Pseudomyotonia | PMT |
Chianina, Romagnola |
||
| Ptosis, intellectual disability, retarded growth & mortality | PIRM | Ayrshire |
||
| Pulmonary Hypoplasia with Anasarca(D) | PHAd | Irish Dexter |
||
| Pulmonary Hypoplasia with Anasarca | PHA | Maine Anjou (1970) |
||
| Rat Tail Calf Syndrome (Dun Dilution Pmel17) | results w/ chance epistatis between dun dilute and HY |
|||
| Recto-Vaginal Constriction | RVC | Jersey |
||
| Spherocytosis | B3 | Wagyu |
||
| Spinal Dysmyelination | SDM | Brown Swiss |
||
| Spinal Muscular Atrophy | SMA | Brown Swiss |
||
| Tibial Hemimelia | TH |
Shorthorn |
||
| Tibial Hemimelia | TH |
Galloway (1974) |
||
| Weak Calf Syndrome isoleucyl-tRNA synthetase (IARS) | IARS | Wagyu (Australia, Japan) |
||
| Weaver Syndrome | W | Brown Swiss |
||
Beta Casein -- (A1/A2): The original (wild type) is A2. The mutation is A1, and it probably mutated about 8000 years ago, in ancestors of the Friesian breeds of cattle. Read about Cow's Milk, A2 beta casein and lactoferrin, which naturally fights viruses like covid, here.
Beta-Lactoglobulin -- (BLG) - BLG is a significant whey protein in cows milk associated with milk yield. This is usually the only whey protein we test for in dairy cattle. Cows with the beta·lactoglobulin genotype of AA can produce more milk that is higher in protein; with less butterfat.
 Breed Base Representation (BBR) is a DNA test to establish relative genetic purity or breed makeup of an animal. There are not BBR tests available for very many breeds yet. "Since the 1800s, breed registries have been established for livestock species to maintain the purity of breeds and to document the ancestry of animals. However, a significant number of animals are unregistered with no or incomplete pedigree data and uncertain ancestral breed origin. Although many heritage livestock breeds are “at risk” on the basis of the number of fullblood breeding females in a breed registry, there is often also a reservoir of unregistered animals that may belong to the same breed. However, due to the missing pedigree it is not possible for breed societies or herd books to include those animals in their closed herd books for pure animals." In 2018 a genetic SNP test was developed to unequivocally determine the breed origin of cattle without pedigree data. This test opens up the possibility to incorporate animals without pedigree data in the breed registry that turn out to be purebred based on the test results. ~ BBR test developers in the Netherlands. Read more here. Photo courtesy of Tim O'Donnell, Dexter Corner Miniature Jerseys.
Breed Base Representation (BBR) is a DNA test to establish relative genetic purity or breed makeup of an animal. There are not BBR tests available for very many breeds yet. "Since the 1800s, breed registries have been established for livestock species to maintain the purity of breeds and to document the ancestry of animals. However, a significant number of animals are unregistered with no or incomplete pedigree data and uncertain ancestral breed origin. Although many heritage livestock breeds are “at risk” on the basis of the number of fullblood breeding females in a breed registry, there is often also a reservoir of unregistered animals that may belong to the same breed. However, due to the missing pedigree it is not possible for breed societies or herd books to include those animals in their closed herd books for pure animals." In 2018 a genetic SNP test was developed to unequivocally determine the breed origin of cattle without pedigree data. This test opens up the possibility to incorporate animals without pedigree data in the breed registry that turn out to be purebred based on the test results. ~ BBR test developers in the Netherlands. Read more here. Photo courtesy of Tim O'Donnell, Dexter Corner Miniature Jerseys.
Calpain 316 / 530 Calpain 316 and Calpain 530 are genes associated with increased meat tenderness.
Fertility-Associated Antigen (FAA) test is an objective measure of a bull's fertility. Backed by university research on thousands of bulls, the ReproTest is a test for Heparin Binding Protein which was found to be an indicator of fertility. Positive bulls are 16–19% more fertile than the average.
Hair Shedding is an EPD, meaning, it is an (expected progency difference) inherited ability to shed hair quicker in spring. Not a DNA test (yet). Selection for this trait can help predict shedding in progeny, expressed in units of hair shed score, with a lower EPD being more favorable indicating a sire should produce progeny who shed their winter coat earlier in the spring. Selection for this trait should improve the genetic potential for a sire’s progeny to shed off earlier increasing the environmental adaptability of cattle living in heat stressed areas and producers grazing endophyte-infected (hot) fescue.
Kappa-casein (KCN) The 2 most common Kappa-casein variants are A and B. The A variant and AA genotype are associated with higher milk production. The B variant and BB genotype are associated with increased milk protein and casein content, and better cheese yield.
Leptin Test for Beef Cattle. Leptin is a hormone that regulates feed intake, energy expenditure and whole body energy balance. A SNP in the Leptin gene has been associated with several economically important traits including milk production, weaning weight, backfat, marbling, quality grade, yield grade, dry matter intake, and days on feed.
Mad Cow Disease in Cattle: Resistance vs Susceptibility to Bovine Spongiform Encephalopathy (BSE): After the first case of BSE in the United States was diagnosed in December 2003, an enhanced BSE surveillance program was established on June 1, 2004, based on the use of a rapid screening test. The blood DNA test is capable of rapid identification of cattle possessing genotypes associated with phenotypic expression of Mad Cow disease. This DNA tool may be useful for import & export, as well as to the domestic cattle industry and to consumers and their health.
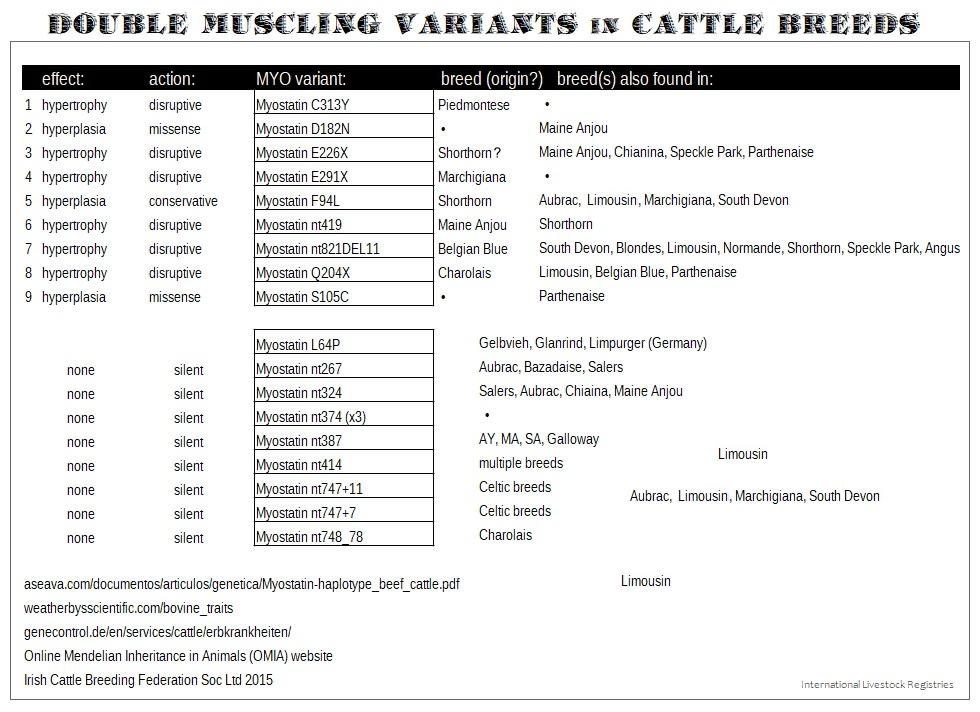
Myostatin - Double Muscling (DM or MYO). Myostatin mutations appeared relatively recent in history and were spread widely with beef breeders selecting for increased muscle. Normal cattle (wild type) have active myostatin. There are several Myostatin variants identified in cattle. They are still not totally understood. But different DM mutations originated in different breeds. Some are dominant and some are recessive; but most display incomplete penetrance, interact with other genes, and with each other when combined. Selection pressure has affected their phenotypic expression. MYO mutations reduce or stop normal myostatin protein production, causing unregulated muscle growth. Different myostatin variants produce different phenotypes, combinations & degree of symptoms. There are pros & cons to raising cattle that are double muscled. Read more here.
Parentage Verification (PV) Proof of Parentage Genotyping, or Genetic Marker Report is done by typing & comparing an offspring's DNA to its parents' DNA. Performed in many genetic testing labs, not all PV tests are compatible with each other, and may not work across multiple registries for the same breed.
Polled: There are several polled mutations, and are dominant over (wild type) horned trait. The inheritance of polledness (the absence of horns) is somewhat complicated, and all polled mutations probably have not been identified. Polled cattle are beneficial in many herds from both an economic and animal welfare point of view. Bruising of other cattle and injuries to handlers due to horns is eliminated, and the trauma of dehorning is avoided (paste de-budding baby calves however is not stressful). Polled variants have been seen in history as far back as 8000 years ago, when some of the earliest evidence of polled domesticated cattle were in today's Slovakia and Germany regions. Read more about polled, scurred & horned traits in cattle here.
Scurred: Inheritance of scurs is not completely understood, but here is what we know so far: Scurs are horn-like tissue attached to the skin--not to the skull as with horns. Scurs can be anywhere from very small to the size of small horns. The scur gene is separate from the polled gene. Scurs (obviously) can only be seen (expressed) in polled cattle. The presence of scurs indicates heterozygous polled (Pp) cattle (meaning, they carry 1 recessive allele for horns). Scurs are suppressed in homozygous polled (PP) cattle. So, if you have a bull with scurs, he would be heterozygous polled, not homozygous polled, so he shouldn't need to be DNA tested for that. Scurs occur more frequently in males than in females. The theory is: scurs may be sex linked, and dominant in bulls. Scurs may be recessive in females. Cows may need to carry two copies of the scur allele to grow scurs; bulls may need only one copy to grow scurs. Research is still ongoing.
Slick Hair: Studies at Brooksville, Florida led by animal scientist Chad Chase have shown slick-haired animals to maintain internal body temperatures at least 1° Fahrenheit lower in summer heat than cattle with normal hair coats. In high production commercial dairy herds especially, heat stress can be an annual issue. Addressing heat stress from a genetic perspective presents a longer term solution. Relatively new on the breeding scene is breeding for the Slick gene in Holsteins. It produces a shorter and smoother coat. This is a gene with dominant heritability (like the polled gene) so that it makes it easier to introduce it into a population. Sires are now available for carriers of the Slick gene. Slick animals in the tropics have been found to have 30% more sweat gland areas and 1.6 degrees Fahrenheit lower surface temperature. University of Florida research shows Slick gene cows, 60 to 90 days in milk, produce 10 lbs. more milk per day in hot environments. And the calving interval for Slick gene cows was 30 days shorter than for normal Holsteins. Thermo Regulatory Genetics
![]()
Coat
Color Genetics
read more about general coat color genetics in cattle here
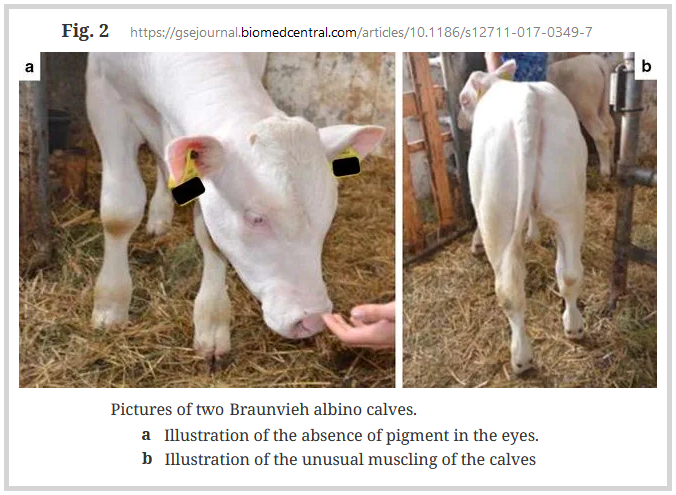
Albinism or oculocutaneous albinism (OCA) is a congential absence of pigment in all parts of the body due to a non-functional form of the enzyme tyrosinase. There may be a number of mutations involved in albinism.
![]()
 Dilution (B) - Dexter Dun on the Brown locus. The Dun coat color in Irish Dexter cattle is a dilution of black pigment (eumelanin) caused by a recessive mutation in the gene tyrosinase related protein 1 (TYRP1), also known as the Brown locus. Black hair is diluted to shades of dark brown to golden. Red pigment is not affected or diluted by this mutation. A second test for red/black pigment (MC1R or Extension gene) may be needed to determine the complete coat color genotype.
Dilution (B) - Dexter Dun on the Brown locus. The Dun coat color in Irish Dexter cattle is a dilution of black pigment (eumelanin) caused by a recessive mutation in the gene tyrosinase related protein 1 (TYRP1), also known as the Brown locus. Black hair is diluted to shades of dark brown to golden. Red pigment is not affected or diluted by this mutation. A second test for red/black pigment (MC1R or Extension gene) may be needed to determine the complete coat color genotype.
Dilution (DUN or SILV) mutation on Pmel17 gene - Incomplete dominance. A dilution gene that causes a black animal to be diluted to a grey color. Heterozygotes show moderate dilution of black to grey, and homozygotes are a light silver. It is less obvious in red cattle, but they may appear orange with 1 copy, and yellow with 2 copies. Sources are unclear if there is more than one mutation for this type of dilution, but it can be epistatically affected with an HY mutation and occasionally produce a less hardy calf with a rat tail. The Hereford breed got yellow dilution from insusion of Simmental, namely Titan 23D. (Fleckvieh, Galloway, Gelbvieh, Murray Grey, Scottish Highland, Simmental, Texas Longhorn)
Dilution (SILV) Incomplete dominance. Some sources cite that Charolais SILV dilution is a unique mutation, some do not. Charolais cattle are genetically red, but most of them possess two alleles for dilution, which results in their typical white coat color. Heterozygote (crossbreds) exhibit strong Charolais dilution of black to light grey, or red to light cream. There may be a few fullblood red Charolais without the Charolais dilution. (Charolais, Blonde d'Aquitaine)
Dilution - Another mutation that causes removal of red pigmentation, with a reduced effect upon black pigment, similar to an agouti type effect; more pronounced in underpinning. Results from Tyrosinase related protein (TYRP1) dilution gene, when homozygous makes dun (an H424Y mutation). (Brahman, Brown Swiss, Chianina)
 Dilution: Heterochromia Irides (HI) “White Eye” is a rare and harmless ocular pigmentation abnormality. White Eye was first noticed in a commercial black Angus cow in the 1980s. Cattle affected by either HI or OH have eyes with irises that are pale blue, grey or gold. In some cattle their hair coats look sun bleached, or diluted. Although they are two separate genetic mutations, HI and OH exhibit similar phenotype.
Dilution: Heterochromia Irides (HI) “White Eye” is a rare and harmless ocular pigmentation abnormality. White Eye was first noticed in a commercial black Angus cow in the 1980s. Cattle affected by either HI or OH have eyes with irises that are pale blue, grey or gold. In some cattle their hair coats look sun bleached, or diluted. Although they are two separate genetic mutations, HI and OH exhibit similar phenotype.
Dilution: Oculocutaneous Hypopigmentation (OH) was recognized as a separate variant from HI in November of 2015. OH was found in Angus & Black Simmental cattle. It's been traced back to a foundation Angus bull Sir WMS Warrant (AAA 9196894) born in 1978, used in the development of black Simmental. Because there is not much difference in the expression of either one, OH is often confused with HI. Both are a simple recessive mutation that cause a harmless cosmetic phenotype. Affected calves have eyes with irises that are pale blue around the pupil with a tan periphery. Their hair coats may have a slightly bleached color. Affected cattle are healthy, functional and physiologically normal.
![]()
Extension Coat Color (CC) The most basic black / red coat color in cattle is determined by the Melanocortin 1 Receptor (MC1R) gene, also called Extension, which controls the production of black (eumelanin) and red (phaeomelanin) pigments. The MC1R DNA test identifies 4 gene variants that affect the coat color of both beef and dairy cattle. The four alleles of the MC1R gene in order of dominance are
Dominant black (ED) is dominant to the other three alleles and animals with ED are black.
Wild type red (E+) produces cattle with red coloration that may appear darker towards the extremities of the body, especially as the animal ages.
Recessive Red (e) Two copies of the recessive red (e) allele result in red color.
Black Red "Telstar" (EBR) found in Holsteins, is a variant that masks the effects of the basic MC1R (Extension) alleles. It results in red & white color at birth which changes to black & white at a young age, usually by 3-6 months.
Dominant Red Coat Color, or Dominant Variant Red (DR)—red that is dominant over black. The Dominant Red gene is independent from MC1R. But Dominant Red, as the name implies, is a dominant red color that overrides black in Holstein cattle. The mutation of dominant Variant Red was traced back to a registered Holstein cow, Surinam Sheik Rosabel-Red. Neither of her parents carried the recessive red gene that was the only known source of red & white Holsteins at that time. DR and MC1R are located in different parts of the genome and are passed on independently. In 2013, the causative gene mutation for the AD+ trait was isolated. Variant Red has the benefit of superseding the traditional MC1R when present, and one or two copies of the gene will be expressed with a red phenotype. (Holstein)
"Red Charlie" variant is named after SV Red Charlie, the Simmental bull it has been traced back to. It was recently identified and is another mutation of MC1R. Red Charlie is a red that is dominant over black when in homozygous form. Therefore, a bull can test homozygous black, but if he is also carrying heterozygous Red Charlie variant, he is in effect, the same as a heterozygous black bull when bred to another Red Charlie carrier. "Red Charlie" was passed down mostly through Remington Lock N Load offspring. (Simmental)
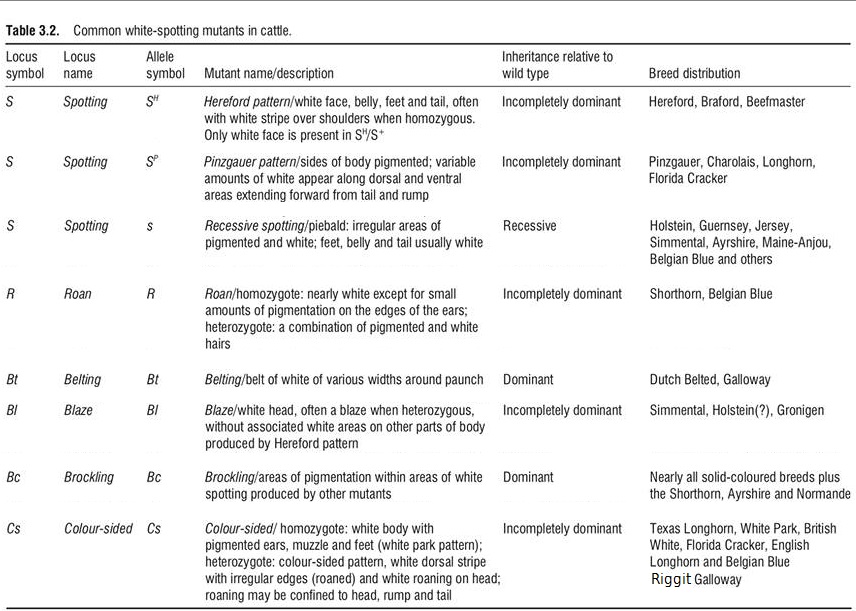
Spotting (S). Spotting is recessive to solid colored coats. When there are only a few spotted cattle in breeds, solid cattle can hide the recessive spotting gene for many generations. The major spotting variants are shown in the table below:
![]()
Breed-Specific
Genotypes
most of
these mutations are recessive and cause undesirable abnormalities in specific
populations
Alopecia (AL) is a simple lethal recessive defect in Normande cattle. Normandes are a French breed, and there are not many fullbloods here in North America. The origin of this defect goes back in particular to Solingen/ Saintyorre, but is found in frozen semen sold in N.A. in 2 bulls: Insoumis and Honess. Synetics
Arachnomelia Syndrome-BS (AS) Spiderleg is a simple lethal recessive defect in Brown Swiss cattle. It is characterized by stillbirth, abnormal skull, reduced body weight, shortening of the spine, and long thin limbs. Caused by a mutation in the SUOX gene. Brown Swiss.
Arachnomelia Syndrome-SM (AS) Spiderleg is a simple lethal recessive gene defect in cattle. Affected animals are born with elongated limbs and facial deformities resulting from aberrant bone development. Caused by a 2-bp deletion in MOCS1 protein. Found in German & Austrian dual purpose Fleckvieh (Simmental) cattle, traced to a daughter of the Simmental bull, Semper. There were 3 popular descendents, one was a bull named Romel, who sired more than 40,000 cows himself. (Fleckvieh)
Arthrogryposis syndrome is ankylosis of the limbs, usually combined with a cleft palate and other growth deformities, particularly in Charolais. At birth, affected calves exhibit joints fixed in abnormal positions and frequently have scoliosis and kyphosis. They are usually unable to stand or nurse. Muscle changes, notably atrophy, have also been seen. Changes may also occur in the spinal cord with necrosis of neurons and lesions of the white matter. Athrogryposis has more than one etiology and pathologic entity. Analysis of pedigrees and matings that produced affected calves revealed that the arthrogryposis syndrome in the Charolais is genetic in origin and caused by an autosomal recessive gene with complete penetrance in the homozygous state. (Charolais)
Arthrogryposis syndrome can also occurs in all breeds of cattle from non-genetic causes... Teratogens identified as causing arthrogryposis include plants such as lupines (anagyrine as the toxic agent) that are ingested by pregnant cows between day 40 and 70 of gestation. Prenatal viral infections with the Akabane @ 451) or bluetongue virus @ 520) can also cause arthrogryposis.
Arthrogryposis Multiplex (AM) (Curly Calf Syndrome) This defect was first noticed by producers in early 2007; a huge mutation (23,000 base pairs; the mutation was found with only 2 months of research). AM affected calves are missing an essential protein that allows communication between nerves and muscle tissue, and the calf (which fails to move in utero) is born under-developed, with atrophied muscles, a twisted spine and legs with the joints of all 4 limbs fixed. Traced through GAR Precision 1680 (1980, right) to his maternal grandsire, Rito 9J9 of B156 7T26. (Angus)
Blindness in Normande cattle. A reverse genetic approach identifies an ancestral frameshift mutation in the RP1 gene, causing recessive progressive retinal degeneration.
Bovine Congestive Heart Failure (BCHF) is an untreatable, fatal condition prevalent in feedlot cattle in the Western Great Plains of North America. A team of researchers from the USDA's Meat Animal Research Center in Clay Center, Nebraska (USMARC) and University of Nebraska-Lincoln (UNL), collaborating with scientists from MatMaCorp, a developer of diagnostic systems for science, agriculture, and medicine, have identified two major genes, ARRDC3 and NFIA, associated with BCHF in beef feedlot cattle. MatMaCorp has translated the newly identified genetic risk factors into a new genetic test that is already being used to inform selective breeding and animal health management. Jan 15, 2020.
Bovine Leukocyte Adhesion Deficiency (BLAD) is a simple lethal recessive gene defect first identified in the early 1980’s. Calves infected with BLAD will have poor health, suffer from stunted growth and reoccurring infections and usually die around 2-4 months of age. BLAD affects white blood cell function which causes extreme susceptibility to infection. The white blood cells of the affected animal fail to attach to the cells lining the blood vessels, an essential step in their migration to the point of infection to destroy invading pathogens. BLAD carriers have a mutation in the CD18 gene (Shuster et al., 1992, Proceedings of the National Academy of Sciences of the United States of America, 89:9225-9229). BLAD status can be determined by using a PCR (Polymerase Chain Reaction) DNA test. The BLAD mutation was traced back to Holstein cattle descended from Osborndale Ivanhoe (1952). Notable carriers included Penstate Ivanhoe Star and Carlin-M Ivanhoe Bell. At one point, 15% of bulls and 8% of cows in the breed were carriers. (Holstein)
Brachyspina (BS) is a simple lethal recessive defect in Holstein cattle. It is characterized by early abortion. Holstein sires that were BS carriers used heavily include Bis-May Tradition Cleitus, Ramos, Rothrock Tradition Leadman, Sandy-Valley Bolton-ET, Silky Gibson-ET, and Wa-Del Convincer-ET. (Holstein)
Cerebellar hypoplasia and abiotrophy. Eight breeds of cattle have been reported to be affected by CA or CH. The 2 genetic conditions are similar in diagnosis. CA is a more common problem in some breeds of cats, dogs and the Arabian horse. These conditions are rare in cattle; cited in a Polled Hereford bull in Australia, and some Holstein cattle in Southern Brazil.
Chediak-Higashi Syndrome (CHS) is a
bleeding disorder in cattle with a light coat colour caused by a mutation
on the LYST gene. The condition causes depressed immunity. The first sign is usually excessive bleeding from the umbilical cord at birth. Check list of original foundation imported carriers here. (Wagyu)
Claudin 16 Deficiency (CL16) or Renal Tubular Dysplasia is found in Black Wagyu. It is an autosomal recessive, considered lethal, usually resulting in death by six years of age. Overgrown hooves, diarrhea, retarded growth, and eventually kidney failure are indicators.
Check list of original foundation imported carriers here. (Wagyu)
Citrullinemia is a simple lethal recessive gene defect. Dairy cattle inheriting two copies of the defective gene die within 5 days of birth due to their inability to eliminate ammonia. Affected calves present with ataxia, aimless wandering, blindness, head pressing, convulsions and death. A big Holstein bull named Linmack Kriss King born in 1965 was the source of the citrullinaemia mutation. He was exported from Canada to England where he was renamed MMB Linmack. Linmack was exceedingly popular as a proven sire in UK, Australia, and especially New Zealand. In New Zealand he supposedly had many sons and grandsons in AI studs, where 1000+ daughters were classified excellent. Due to testing, citrullinemia is becoming rare. (Holstein)
Complex Vertebral Malformation (CVM) is a simple recessive lethal syndrome found in Holstein cattle that will increase the rate of embryonic loss and number of stillbirths. Among the pregnancies that inherit the defective gene from both parents, 80% of the embryos will be lost during first 3 months of gestation, and losses continue to progress afterwards with only a few surviving to near term. A stillborn CVM calf will have a shortened neck due to malformed vertebra, fused ribs on the right side, and contracted fetlocks. The mutation was discovered in Denmark. Genealogical research identified Carlin-M Ivanhoe Bell (registration number 1667366), an elite Holstein-Friesian bull born in 1974, to be the main ancestor of cattle carrying this mutation. Because of the superior lactation performance of his daughters, Ivanhoe Bell was used extensively for two decades. Carriers of the CVM mutation exist in Holstein cattle populations worldwide. (Holstein)
Congenital Erythropoietic Porphyria (CEP) see also: Protoporphyria (AKA Porphyrinuria, Pink tooth, Osteohemochromatosis) CEP was first reported in South African Shorthorn cattle; however, most cases have since been reported in the Holstein breed and the English Longhorn. It is inherited as a simple autosomal recessive. Heterozygous animals are normal, but homozygous recessive animals are affected at birth with reddish brown discoloration of the teeth, bones, and urine that persists for life. CEP has been recognized since 1962 in the USA, Canada, Denmark, Jamaica, England, South Africa, Australia, and Argentina. This broad geographic distribution suggests that a number of mutations cause this disease worldwide and probably exist in most meat-producing animals, especially cattle, swine, and sheep. A similar disease, Erythropoietic protoporphyria (Bovine EPP), or, bovine congenital Erythropoietic protoporphyria (BCEPP), also causes photosensitivity with possible seizures, and is seen mostly in the Limousin breed, occasionally described in the Blonde d'Aquitane breed.

Contractural Arachnodactyly (CA) – Fawn Calf Syndrome Recognized by 2004 among Angus reported in Australia–Subtle, non-lethal, calves appear hunched or crouched, less extension of upper limb joints– Some reported calves trace to Bon View Bando 598, born in 1988, or to his great-grandsire, Premier Independence KN, born in 1981. (Angus, Murray Grey)
Crooked Tail Syndrome (CTS) In addition to the deviation of the tail, cattle with CTS have general retarded growth by about 1 month of age, abnormal skull shape with a shortened broad head, and extreme muscular hypertrophy. Although the defect is not lethal itself, the most severe cases (about 25%) are euthanized on welfare grounds. The surviving 75% cause important economic loss as a result of growth retardation and carcass depreciation. CTS carriers are smaller, stockier, heavier muscled, and they have a finer bone and more well-sprung ribs. CTS carriers are about twice as likely to be selected as elite sires than their noncarrier siblings, which contributed to the rapid increase in the CTS mutation in Belgian Blue cattle. (Belgian Blue)
Although the defect is not lethal itself, the most severe cases (about 25%) are euthanized on welfare grounds. The surviving 75% cause important economic loss as a result of growth retardation and carcass depreciation. CTS carriers are smaller, stockier, heavier muscled, and they have a finer bone and more well-sprung ribs. CTS carriers are about twice as likely to be selected as elite sires than their noncarrier siblings, which contributed to the rapid increase in the CTS mutation in Belgian Blue cattle. (Belgian Blue)
Crop Ear (CE) This is a cosmetic mutation in Scottish Highland cattle that causes cropped ears that look frostbitten. The mutation has a dominant expression of incomplete penetrance; so, a homozgous crop ear calf will have more obvious cropping than a heterozygous crop ear calf will. With their long hair, heterozygous crop ear calves may not be noticeable. The earliest common ancestor was a sire born in 1943 (I haven't yet found out what the bull's name was).
Understanding Haplotypes: When the causative gene of a condition is not known, analysis of all genotypes in the breed may be able to identify a short section of the genome, known as a haplotype, that is expected to include the causative gene. While very valuable in controlling the spread of undesirable genes, identifying carrier animals based on haplotypes is not as accurate as results from a specific gene test. Since haplotype results are not 100% accurate, they are presented as carrier probability values ranging from 1% (likely free) to 99% (likely a carrier).
Dairy Fertility Haplotypes; Cow fertility is an important component of longevity, because infertility is presently the most common reason for culling in most dairy herds. Geneticists began searching dairy breeds for gene pairings that never showed up in living cattle. The assumption is that such a “haplotype” (genomic marker) must therefore be lethal when homozygous —either conception fails or embryonic death occurs. Dairy fertility haplotypes' mode of inheritance are autosomal recessive. Abortions occur within predictable time lengths. There is little known about these dairy cattle mutations because affected calves are aborted. It is expected that more haplotypes will be identified each year. UPDATES: Haplotypes BH1 and JH2 were discontinued in December 2018 (VanRaden and Null, 2018).
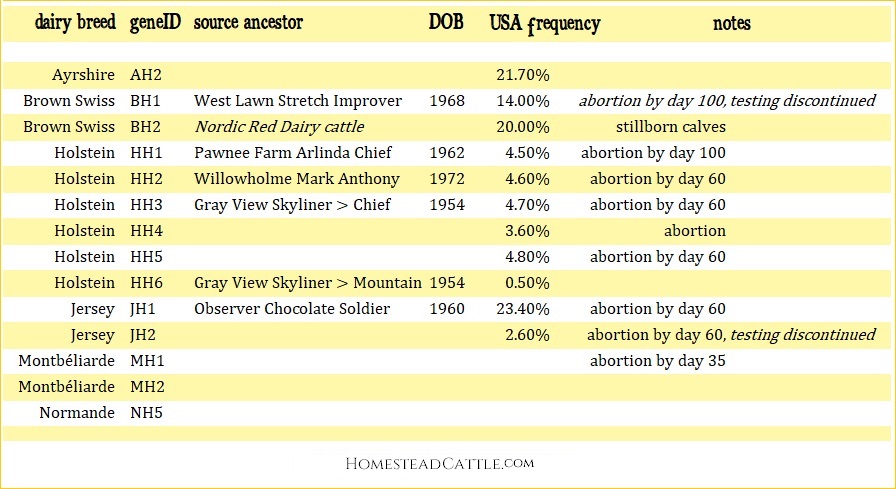
Dairy Health Trait Haplotype DNA Panels: CLARIFIDE® Plus claimed to be the first commercially available dairy genetic evaluation specifically designed for wellness traits in U.S. Holsteins at the time of this writing. This is a panel that predicts risk for six common diseases that are influenced by genetic resistance vs. susceptibility. Mastitis, Lameness, Metritis, Retained Placenta (RP), Left-Displaced Abomasum (LDS) and Ketosis, plus will identify and differentiate homozygous vs. heterozygous polled. Currently, Holstein producers can order the CLARIFIDE Plus test panel through Zoetis; Dairy Haplotype Fact Sheet. (2019)
Montbéliarde MH haplotype. Previous studies (Schwarzenbacher et al., 2016a,Schwarzenbacher et al., 2016b; Michot et al., 2017; Fritz et al., 2018) have been outdated, showing they focused on invalid candidate causal mutations
Normande NH7 homozygous haplotype deficiency (HHD) Recessive Inheritance of CAD p.Tyr452Cys in Normande A missense mutation (p.Tyr452Cys) in the CAD gene with incomplete penetrance compromises reproductive success in French Normande cattle. Haplotype NH7 Compromises Conception Rate in Normande Cattle. we suspect that gradual embryonic loss occurred over the length of gestation, rather than abrupt mass loss in the first month after fertilization or specific trimesters.
Homozygosity mapping is an efficient approach to map such recessive deleterious alleles.
The genome of 77,815 Normande cattle were scanned with different Illumina SNP chips (Illumina Inc., San Diego, CA) to map recessive embryonic lethal mutations using homozygous haplotype deficiency. 2 novel haplotypes were detected on chromosomes 11 and 24 but did not confirm 6 previously reported haplotypes. The one on chromosome 11 showed a marked reduction in conception rates and moderate decrease in nonreturn rate in at-risk versus control mating, supporting late embryonic mortality. The only known homozygous NH7 cow had extremely poor udder conformation, suggesting a potential role of CAD in udder development. Despite being alive and apparently healthy and fertile, this animal had very poor udder morphology (scored among the lowest 1% of animals with same sire). Fritz et al., 2013
Deficiency of Uridine Monophosphate Synthase (DUMPS) is a disease of Holstein cattle resulting from a simple lethal recessive gene defect that causes a failure in the synthesis of DNA. Embryos inheriting two copies of the gene defect results in early embryonic mortality during implantation in the uterus and possibly fetal mummification; most are lost around day 40 of pregnancy. DUMPS carriers have a mutation with loss of an AvaI site at codon 405 (Schwenger et al., 1993, Gene Genomics 16:241-244). Major carriers of this defect were Happy Herd Beautician and Skokie Sensation Ned. DUMPS is now getting closer to being eradicated. (Holstein)
Developmental Blindness (DB) is apparently an autosomal recessive novel mutation (isolated November 21, 2023). Cattle develop blindness at approximately 9—12 months of age. Testable at Neogen for $20 as a standalone or $13 as an add-on. Search carrier cattle here. (Hereford)
Developmental Duplication (DD) (polymelia) –DD is anabnormalitythat has been long-observed in Angus cattle, previously thought to be caused by conjoined twins or other anomalies during fetal development. This abnormality was recently found to be a simple inherited recessive genetic condition (with incomplete penetrance) passed through certain lines of Angus cattle. Unlike some of the other simple recessive traits, not all animals who are homozygous recessive (DDA) will express the condition. Animals affected with this condition can sometimes be born with an extra limb or part of an extra limb (a condition referred to as polymelia). Bloodline: Bull named B/R New Design 036, born 1990 as the major carrier; but the mutation was traced back to his grandsire, Ken Caryl Mr Angus 8017 (1977). (Angus).
Digital Subluxation (DS) Though there are 2 original mutations (slightly different) which occured in completely separate populations, the resulting diseases are the same. In both mutations, the DS is in close proximity on the chromosome to the allele that causes another defect; Pulmonary Hyperplasia with Anasarca (PHA). A parent that is a carrier for both, is therefore likely to pass on both mutations to its progeny. When both the DS and the PHA mutations are present, the PHA can impact the phenotype (physical appearance) of a DS Carrier. (Chianina-DSC, Shorthorn-DSH)
![]()
DWARFISM. Since the eighteenth century, references have been made to the “monstrous bulldog calf.” The first recognized scientific report on BD was published in 1904, describing it in the Dexter breed as “cretinism in calves” and raising the hypothesis that it was a hereditary anomaly resulting from Mendelian inheritance. It was indeed, and was one of the first genetic understandings made in livestock. In addition to this well-documented occurrence in the Dexter breed, several other BD mutations have been identifed in other breeds. And there are other types of dwarfism found in cattle that are not Bulldog BD types. Below are the dwarfisms known about in Homestead and Miniature breeds of cattle.
 Dwarfism BD1 Bulldog Chondrodysplasia CHO (Irish Dexter): "Bulldog" calves are usually aborted in mid to late gestation. Two different mutations in the aggrecan (ACAN) gene, on chromosome 21 of the Bos taurus genome, have been identified in generalized chondrodysplasia in Dexter cattle. The common one is the BD1 mutation which originated long ago in Irish Dexters; and is an insertion mutation (2266_2267insGGCA). (Cavanagh et al. 2007)
Dwarfism BD1 Bulldog Chondrodysplasia CHO (Irish Dexter): "Bulldog" calves are usually aborted in mid to late gestation. Two different mutations in the aggrecan (ACAN) gene, on chromosome 21 of the Bos taurus genome, have been identified in generalized chondrodysplasia in Dexter cattle. The common one is the BD1 mutation which originated long ago in Irish Dexters; and is an insertion mutation (2266_2267insGGCA). (Cavanagh et al. 2007)
Dwarfism BD2 Bulldog Chondrodysplasia CHO: was discovered in New Zealand upgraded Irish Dexter cattle descended from Meadowpark Charles but its origin was traced back to his granddam, a small Red Poll X Jersey cow. BD2 is not found in North America. BD2 involves a transition mutation (-198C>T) (Cavanagh et al. 2007)
 Dwarfism BDz Bulldog Chondrodysplasia. This variant was isolated in October of 2018 in Germany[1], in Miniature Zebu cattle from North America, where it originated. We do not know yet how prevalent this mutation is in America--how long it has been around, or what bloodline(s) it exists in. This mutation is an autosomal recessive gene, therefore it does not cause dwarfism in carriers. Although carriers are not affected, homozygous BD calves are aborted, which if found, indicates both parents are carriers. Testing (at the time of this writing) costs $75 at UC Davis.
Dwarfism BDz Bulldog Chondrodysplasia. This variant was isolated in October of 2018 in Germany[1], in Miniature Zebu cattle from North America, where it originated. We do not know yet how prevalent this mutation is in America--how long it has been around, or what bloodline(s) it exists in. This mutation is an autosomal recessive gene, therefore it does not cause dwarfism in carriers. Although carriers are not affected, homozygous BD calves are aborted, which if found, indicates both parents are carriers. Testing (at the time of this writing) costs $75 at UC Davis.
Dwarfism BD(x) Bulldog Chondrodysplasia. Unnamed mutation; currently under investigation; what appears to be another semi-dominant bulldog chondrodysplasia in a North American midwestern herd of miniature cattle, tested negative for BD1, BD2 and BD(z). Research is ongoing. It is speculated that it is a novel BD gene that interacts with BD1 like BD2 does. More information should be forthcoming this year, 2020.
Dwarfism BD(n) Bulldog Chondrodysplasia CHO found in the Nelore /Nellore breed (right), a standard size Zebu breed that is most popular in Brazil. There are a few Nellore in North America.
 Dwarfism D2 (Long-Nosed dolichocephalic proportionate Dwarfism). An autosomal recessive gene. This is not a bulldog type dwarfism. It is a relatively recent re-emergence of dwarfism in Angus beef cattle. In 2003, an Angus AI sire born in 1997, High Valley 7D7 of 4G9, was identified as the first known carrier of long-headed dwarfism. This carrier bull was not widely used, so carriers today are rare. Calves are normal looking at birth, but as they age, when compared to herdmates, they are shorter in stature because the long bones in the legs fail to grow. Their legs mature short but the head is of normal size. These dwarves appear to have normal fertility. (Angus)
Dwarfism D2 (Long-Nosed dolichocephalic proportionate Dwarfism). An autosomal recessive gene. This is not a bulldog type dwarfism. It is a relatively recent re-emergence of dwarfism in Angus beef cattle. In 2003, an Angus AI sire born in 1997, High Valley 7D7 of 4G9, was identified as the first known carrier of long-headed dwarfism. This carrier bull was not widely used, so carriers today are rare. Calves are normal looking at birth, but as they age, when compared to herdmates, they are shorter in stature because the long bones in the legs fail to grow. Their legs mature short but the head is of normal size. These dwarves appear to have normal fertility. (Angus)
Snorter Dwarfism. (Brachycephalic dwarfism) that was common in Hereford cattle in the 1950's, and has probably been eliminated through genetic selection. They were characterized by short faces, bulging foreheads, prognathism, large abdomens, and short legs. They were approximately half normal size. This genetic defect was spread as a result of strong selection pressure for animals with small stature in the 1940s and 1950s. A 1956 survey of Hereford breeders in the USA identified 50,000 dwarf-producing animals in 47 states. Snorther dwarfism caused abnormal sinus bone development with overall dwarfism, and was identified by cattle with shorter muzzles and impaired breathing. They were of low viability and susceptible to bloat. Snorter dwarfism spread mostly from a Wyoming Hereford Ranch and a bull named Superior Mischief 21. From him the mutation was traced back to a bull named St. Louis Lad, born in 1899, and later, from him, to his grandsire, Kansas Lad. Snorter Dwarfism may be eradicated by now. [read more: http://herefordtalk.com/thread/3466/dwarfism] (Herefords)
![]()
Erythropoietic protoporphyria (Bovine EPP or CEP) found in Limousin cattle. Also see Protoporphyria.
Factor XI Blood Coagulation Deficiency (F11 haemophilia) found in Holstein cattle. Factor XI is a plasma protein that participates in the blood coagulation process. Heterozygous cattle have decreased levels of factor XI but are usually asymptomatic. The severity of clinical disease varies in homozygous cattle, but they usually show prolonged or repeated bleeding episodes after trauma such as dehorning and hemorrage following venipuncture. There are occasional deaths associated with multiple hemorrhages. This bovine disorder has been known since 1969 when it was first seen in Holstein cattle in Ohio, but mostly spread later from a Canadian Holstein sire.
Factor XI Blood Coagulation Deficiency (F11 haemophilia) found in Wagyu. There are similarities in the clinical symptoms in the Holstein and the Wagyu diseases, however the causative insertion mutations are on different locations of the F11 gene and these variations are considered to be responsible for the differences in severity between the breeds. Tests carried out in Australia and USA have identified the Factor XI deficiency in both Red and Black Wagyu. This implies that the F11 mutation in Wagyu occurred before the segregation into Black and Red breeds. Factor XI deficiency in Wagyu has an autosomal recessive mode of inheritance and is more prevalent in higher marbling lines.
Factor XIII Blood Coagulation Deficiency (F13 haemophilia) found in Wagyu, non-lethal.
Fishy Off-Flavor Milk (FMO3) There is a causative mutation in the FMO3 gene of some cattle that causes fishy off-flavor milk. Affected animals have bodily fluids characterized by a distinct, unpleasant smell of rotting fish. Though not a lethal recessive, this is a highly undesireable attribute. This defect was first seen in Swedish Red and White cattle and can now be found in Ayrshires as well.
Freemartin: This is a gender abnormality in cattle, a condition that occurs in utero in about 90% of all heifers born fraternal twins to bull calves. During embryonic development, the bull's male hormones influence the heifer. Twin bulls are normal, bulls born twin to heifers are normal, and twin heifers are normal. Without a test, many producers simply cull any heifer twin to a bull. Genetic Visions developed a DNA based test to identify the presence of the Y chromosome. Since females are XX and males are XY, a positive Y chromosome test result in a heifer born twin to a bull indicates the freemartin condition. The number of cells containing the Y chromosome will be greatest at birth, but it is a lifelong condition, that can be tested for at any time. The freemartin condition occurs in other livestock species as well. See glossary for info about Freemartins in sheep & goats.
High Altitude Hypoxic Pulmonary Hypertenstion (HAPH) or Bovine High-mountain Disease, Pulmonary Arterial Pressure, Dropsy, High Altitude disease, or Brisket disease - Using diverse U.S. beef cattle genomes to identify missense mutations in EPAS1, and a gene associated with high-altitude pulmonary hypertension. Research was done on HIF2A protein variant diplotypes in 1154 animals from 46 breeds of U.S. cattle, and 6 missense mutations in bovine EPAS1 were identified associated with high altitude PH. MERCK Manual: “Bovine high-mountain disease is a condition that occurs in cattle pastured at high altitude. Low oxygen saturation in the air leads to pulmonary hypertension, with subsequent edema in ventral tissues of the chest and abdomen. Treatment typically requires movement to lower elevation, and prevention through selective breeding for resistant stock is often preferred. Treatment of BHMD can be expensive and unrewarding, so prevention is preferred. Genetic selection through the use of pulmonary arterial pressure (PAP) measurements to select cattle resistant to the effects of hypoxia is a more effective way to control BHMD. Identifying animals highly susceptible to the effects of altitude hypoxia (those with high PAP measurements) and eliminating them from the breeding pool are practical methods to reduce the prevalence of BHMD in a herd. The PAP measurement procedure involves passing flexible polyethylene catheter tubing (1.19 mm internal diameter × 1.7 mm external diameter) through a large-bore needle (12 or 13 gauge, 3.5 in.) inserted into the jugular vein. The catheter is advanced through the jugular vein to the right atrium, into the right ventricle, and then into the pulmonary artery. A normal mean PAP measurement should be 34–41 mmHg. Any animal with a PAP measurement >48 mmHg is considered at risk of developing BHMD and may be a potential genetic carrier and should not be maintained or used in breeding programs at high altitude.”
Holstein Cholesterol Deficiency Haplotype (HCD) is a semi-dominant genetic defect. Heterozygous carriers of the disease show lower levels of cholesterol in the blood than normal animals, and their ability to produce cholesterol is reduced. If inherited homzygous, the animal doesn't produce cholesterol in their cells. Without fat, the starving animal slowly weakens and dies. The oldest known source of this mutated gene is Maughlin Storm (HO.CAN.M5457798). Old (heritage or fullblood) non-Holstein dairy breeds are safe, but modern bred dairy breeds (many of today's dairy breeds have been improved with Holstein genetics) may need to be checked for HCD. (Holstein)
Hypotrichosis (HY) - Hairless Calf Syndrome. Not lethal unless affected calves succumb to elements--several HY mutations have been identified; resulting in abnormal, curly, patchy or balding hair in several breeds of cattle. There is a DNA test for a variant in Polled Hereford that trace to to JR Nick the Butler P183, born in 1982. Other variants have been mentioned in Belted Galloway. At least 13 variations of hypotrichosis have been described in Angus, Ayrshire, Brangus, Holstein, Hereford, polled Hereford, Guernsey, Gelbvieh, Jersey, Normande, Maine Anjou, Charolais, and Simmental cattle and crosses. Most have autosomal recessive modes of inheritance. The "rat-tail" syndrome (RTS) is seen in crossbred (SILV) dilute calves such as Charolais, Hereford or Simmental crossed with black Angus or Holsteins. The calves have short, curly, malformed, sometimes sparse hair, and a lack of normal tail switch development. The affected animals also suffer from disturbed thermoregulation, which impairs their health and growth performance. One study provides evidence that the RTS phenotype results from an epistatic interaction between three independent loci: dilution, extension (MC1R gene) and the RTS locus (many breeds).
 Idiopathic Epilepsy (IE) • Also known as Inherited congenital myoclonus (ICM) • Diagnosed in Herefords (mostly horned) in 2003 • Carriers trace to HH Advance P242, born in 1982, mostly from his widely-used grandson, HH Advance 9012Y (left), born in 1989. Affected calves have seizures, often stress induced–that can last for minutes, up to an hour • Affected calves appear normal when not seizing. (Hereford)
Idiopathic Epilepsy (IE) • Also known as Inherited congenital myoclonus (ICM) • Diagnosed in Herefords (mostly horned) in 2003 • Carriers trace to HH Advance P242, born in 1982, mostly from his widely-used grandson, HH Advance 9012Y (left), born in 1989. Affected calves have seizures, often stress induced–that can last for minutes, up to an hour • Affected calves appear normal when not seizing. (Hereford)
Jersey Neuropathy with Splayed forelimbs (JNS) In November 2020 JNS was officially identified in the Jersey breed. JNS is a recessive genetic anomaly and when inherited from both parents results in a calf that is born alive and appears healthy but they cannot stand. Affected calves exhibit neurologic symptoms with muscle stiffness in the head and neck and they also have front legs that are rigid and spread out in front of their body. At the present time, no gene test is available to identify carriers with complete certainty but a high level of accuracy can be achieved by the analysis of haplotypes (a short section of the genome) for genotyped Jerseys. The CDCB in United States has expanded its current analysis of haplotypes to include JNS. SOURCE of JNS:
Based on an analysis of all genotyped Jerseys conducted by CDCB, the oldest genotyped animals that were identified as carriers of the JNS haplotype are Woodstock Berretta Leslie (JEUSAF3906889, born in January 1995) and her daughter, Woodstock Alf Leslie (JEUSAF3980701, born in December 1996). In terms of how JNS infiltrated the Canadian Jersey population, however, it appears this mainly happened via the high-ranking grandson of Woodstock Berretta Leslie, namely Tollenaars Impuls Legal 233-ET, born in 2004. Table 1 provides a list of those sires that have been identified as a carrier of the JNS haplotype and have at least 50 registered daughters born in Canada. Other than Woodstock LLV Lieutenant-ET, who shares the same maternal granddam as Legal, all other sires in Table 1 are direct descendants of Legal.
A complete list of carrier bulls, with corresponding NAAB codes, is included in the American Jersey Cattle Association (AJCA) Green Book. (Jersey)
Limber Legs (LL) Some calves will be born dead; survivors may appear normal but be unable to stand. Muscles, tendons, ligaments, and joints will be malformed or underdeveloped. The affected calf has little or no control over movement of legs. The calf's legs lack normal muscling, appear loose at the joints, and can be flexed, extended and rotated without difficulty or discomfort to the calf. (Jersey)
Mandibulofacial Dysostosis (MD) resulting in unsurvivable facial deformities; caused by an autosomal lethal recessive genetic mutation. The common denominator (and probable source of this mutation) in carrier cattle is SHF Wonder M326 W18 ET (42991698), identified on Herfnet.com as the oldest Mandibulofacial Dysostosis Carrier (MDC), as well as the following carrier descendants:
NJW 73S W18 Homegrown 8Y (43214852, 1300+ progeny),
NJW 73S W18 Hometown 10Y (43214853, 6300+ progeny),
SHF Access Y90,
K King 400,
GO MS Access C81, GO MS Access E24,
GO King E43, RVF Sweet Pea Shes Real Quiet, GO King F42,
Perks Cato Rummy 8011. Wonder's sire KCF Bennett 3008 M326 tested free. The maternal granddam, SHF Governess 236G L37 (SHF Interstate D03 G80 (P24016302) x GK Lady Banner 563T (P22988426)) was not tested.
If your Polled Hereford does not have Wonder or his maternal granddam in its pedigree, testing would be unecessary. Test available through Neogen. (2020, Polled Herefords).
Mannosidosis - Alpha (a)-Mannosidosis (MA) (Red Angus)
Mannosidosis - Beta (ß)-Mannosidosis • Calves exhibit weakness, incoordination, head-swaying, poor suckling reflex, other nervous system disorders • DNA test available since 1999 • Defect is now rare. (Salers)
Maple Syrup Urine Disease (MSUD) or Neuraxial edema in Herefords is a simple autosomal recessive, meaning that 50% (half) of the offspring from a carrier are expected to inherit the faulty MSUD gene. Carriers are unaffected by the disease. It's when two copies of the MSUD mutation are inherited, one from each carrier parent, that a calf inherits the disease. The calves become lethargic and unsteady by 2-4 days of age, most are recumbent (unable to stand) by 3-5 days and dead by day 7-10. The problem lies with a metabolic pathway that cannot correctly break down the proteins in milk. These proteins then buildup to toxic levels and cause brain damage and liver damage until the calf succumbs. There are no medications or therapies that can reverse this process available at this time. Thiamine can help protect the brain but it can't stop the process and every swallow of milk brings in more protein the calf can't metabolize. Your vet may initially diagnosis the death as one of two things (neither of them are MSUD): 1. Meningitis, 2. Bacterial septicemia. In reality, be warned that it is unlikely that the urine will exhibit the odor of maple syrup for which the condition is named. Using this as a diagnostic criteria should be avoided. Brain or liver samples sent to a diagnostic lab may not get you the MSUD diagnosis either because vets aren't looking for it, labs aren't looking for it yet, and to date there is only 1 lab in the country that can definitively test for it, Neogen GeneSeek. The earliest DNA test for MSUD was available to the public March 6, 2019. As of 07/15/2019, there are over 500 known MSUD carriers, in both horned and polled Herefords, of which over 30 are Miniature Herefords. ~ read more here: H&R Miniature Herefords.
![]()
Mulefoot LRP4 (Syndactyly) is mostly a cosmetic defect, resulting in an animal having only one toe rather than the usual two. It normally affects the front feet, but can affect the rear ones as well. At least 4 known variants occur in Holstein, Angus, Simmental and Charolais.
 Neuropathic Hydrocephalus (NH) (Angus) An affected calf is born near term and has 25-35 pound birth weights. The head (cranium) is markedly enlarged (volleyball to basketball sized). The bones of the skull are malformed and appear as loosely organized bony plates that fall apart when the cavity is opened. The cranial cavity is filled with fluid and no recognizable brain tissue is evident. The spinal canal is also dilated and no observable spinal tissue is found. Single base pair mutation. Source: GAR Precision 1680 (AAA #11520398) (1990). He is the original, spontaneous mutant--his sire and dam both tested NH free.
Neuropathic Hydrocephalus (NH) (Angus) An affected calf is born near term and has 25-35 pound birth weights. The head (cranium) is markedly enlarged (volleyball to basketball sized). The bones of the skull are malformed and appear as loosely organized bony plates that fall apart when the cavity is opened. The cranial cavity is filled with fluid and no recognizable brain tissue is evident. The spinal canal is also dilated and no observable spinal tissue is found. Single base pair mutation. Source: GAR Precision 1680 (AAA #11520398) (1990). He is the original, spontaneous mutant--his sire and dam both tested NH free.
Osteopetrosis (OS) - Marble Bone - Angus & Red Angus, Hereford, Simmental, Limousin. Most recently diagnosed in Red Angus • Past occurrences in Angus, other breeds (NOTE: The Red Angus mutation is not the same as the mutation that causes marble bone in Black Angus cattle. Many are independent mutations in different breeds) • Several prominent Red Angus AI sires were identified as carriers. Analysis of pedigrees plus historical information suggests that the mutation may have originated in the 1950’s. The oldest known carrier bull, as determined by DNA testing, is BKT STRM 751626 (RAAA #8605, born 1964) and other known carriers suggests that the mutation may have occurred at least two generations prior to “751626”. Calves are born dead and often premature. The body may be unusually small and the lower jaw may be undershot. Bones will be solid and thick but lacking a bone marrow cavity. Caused by deletion in a gene identified on chromosome 4.
Progressive Ataxia, Charolais and Blonde d' Aquitaine cattle.
Protoporphyria (Proto) or CEP; is a rare autosomal recessive genetic disease of cattle worldwide that causes photosensitivity with possible seizures, (severe sunburn) due to a deficiency of ferrochelatase, an enzyme that inserts iron into protoporphyrin. • Calves are sensitive to light, sunburn • Calves develop hair loss, sores, fail to thrive • DNA test available since the mid-1990s • Defect largely removed from the population, potential carriers must be tested in order to be registered. Also known as Erythropoietic Protoporphyria (Bovine EPP), or, Bovine Congenital Erythropoietic Protoporphyria (BCEPP). Seen mostly in the Limousin breed, occasionally described in the Blonde d'Aquitane breed. (Limousin, Blonde d'Aquitaine)
Pseudomyotonia (PMT) in Chianina & Romagnola breeds.
PIRM: Ptosis, intellectual disability, retarded growth and mortality was discovered as the Ayrshire hapotype 1. The source was determined to be from a Finnish Ayrshire bloodline imported into USA. The frequency of AH1 presence in the Ayrshire breed is approximately 24%. (Ayrshire)
Pulmonary Hypoplasia with Anasarca (PHA) -- (see also DS) - Maine Anjou. PHA is a huge mutation (23,000 base pairs), and the mutation was discovered after only 2 months of research. DNA test is commercially avail. PHA calves are born dead or die shortly after birth.
Translated, that means small, under-developed lungs (pulmonary hypoplasia) and lots of excess, retained fluid (anasarca). This is also a lethal defect and the calves are usually born dead or calves may also be aborted early. The anasarca is a major problem. Calves can retain so much fluid that an 80 pound calf can weigh 200 pounds. PHA often causes dystocia and may require a C-section.
The mutation probably came over w/ importation of Maine-Anjou to US, but in today's cattle usually traces back back to three popular bulls: two registered Maine-Anjou bulls (Draft Pick, born 1989; AMAA #165,744 and Stinger, born 1985; AMAA #111,205) and a bull registered with the American Chianina Association (Payback, born 1992; ACA #232,907). Subsequent genetic testing suggested a common ancestor of the three bulls. Draft Pick’s maternal great grand sire, Paramount (AMAA # 77; born 1973), a full-bood Maine-Anjou bull exported from England to Canada, was a PHA carrier. Due to incomplete or inaccurate pedigrees or inability to obtain samples from older full-blood Maine-Anjou cattle, the origin of the mutation in Stinger and Payback has not been positively identified. Molecular markers surrounding the gene suggest the French import Dalton (AMAA #15; born 1970) as a common source for Stinger and Payback. In the USA, the Maine-Anjou breed is considered the origin of PHA. In registered Maine-Anjou cattle, Draft Pick is primarily responsible for the widespread dispersion of the mutation, whereas in Shorthorn (impure, or non-heritage) cattle, Stinger is considered primarily responsible. (Maine Anjou)
Pulmonary Hypoplasia with Anasarca (PHAD) -- The Dexter PHA mutation came to the United States and Canada with the importation of a PHA-carrier cow, Woodmagic Wheatear. We don't know if she inherited this mutation from her sire or from her dam, and we don't know how far back it goes. Neither her sire, nor her dam were tested for PHA. To date, the Dexter Cattle Society in the U.K. has not published any testing results for PHA in the Dexter cattle of their country, that we are aware of. Woodmagic Wheatear was an excellent Dexter cow. Her son Aldebaran Priapus was the first Dexter bull collected for AI in Canada. Woodmagic Wheatear was flushed for embryos and those embryos were exported to Australia, as was semen from her grandson, Trillium Chabotte (also a PHA-carrier). Several sons of Aldebaran Priapus were imported from Canada into the United States and used extensively by major breeders. Woodmagic Wheatear was imported into Canada in 1978. Her son, Aldebaran Priapus, was born in Canada in 1982. Aldebaran Priapus' son, Trillium Chabotte, was born in Canada in 1985. We've had the test for PHAD since 2009. Read more here. (Irish Dexter)
Rat Tail Calf w/ Dun Dilution (SILV) - Simmental. (Pmel17) gene. Incompletely dominant. Sources vary whether or not this dilute mutation of coat color dilution is different than the Charolais Dc dilution. Heterozygotes show moderate dilution of black to grey, and homozygotes are a lighter grey. The Ds dilution mutation does not express as obviously in red cattle. This dilute mutation can be epistatically affected by a HY mutation, and be associated with a rat tail calf, which is an undesirable, less hardy calf. (Simmental, Hereford, Gelbvieh; maybe Charolais)

Recto-Vaginal Constriction (RVC) affects Jersey cattle of both sexes. In females it results in the compression of the genital tract and the sphincter or both sexes. Dystocia, udder edema, and excess fibrous tissue on the genital /rectal muscles often accompany this defect. Constriction of the rectum and vagina are such that the arm usually may not be inserted to permit artificial insemination. An episiotomy or Caesarian section is usually required for calving. The condition may be accompanied by hardening of the udder. (Jersey)
Spherocytosis (B3) -- Erythrocyte Membrane Protein Band III Deficiency (Band 3)
is rare. Low birth weight is followed by labored breathing from moderate anemia with spherocytosis, retarded growth and acidosis. It is lethal and death usually occurs within ten days after birth.
(Wagyu)
Spinal Dysmyelination (SDM) is a simple lethal recessive genetic defect in Brown Swiss cattle that is symptomatic from birth. Affected animals are never able stand and die within a week. SDM is a neurodegenerative genetic disease. The homozygous recessive affected calf cannot stand, with symptoms that include lateral recumbency with slight to moderate opisthotonos, body tremor, and spastic extension of the limbs. Attempts to rise and limb movements are absent; however, the animals remain alert to their surroundings, and spinal reflexes are normal or slightly increased. SDM carriers have an R560Q substitution with loss of function (Thomsen et al., 2010 May, Neurogenetics, 11(2): 175–183). (Brown Swiss)
Spinal Muscular Atrophy (SMA) is a simple lethal recessive gene defect causing a neurodegenerative wasting disease in Brown Swiss cattle. Symptoms usually begin at around 3 to 4 weeks of age with weakness of the rear legs. Terminal stages are marked by weakness in all four limbs, severe muscular atrophy and inability to stand. Once the calf is no longer able to stand and it affects the major organs, death usually occurs within a few days from pneumonia. Affected animals usually die by 6 to 8 weeks of age. The causative gene SMN has been mapped at the very distal end of BTA24 (Krebs et al., 2007, Proceedings of the National Academy of Sciences of the United States of America, 104:6746-51 ). (Brown Swiss)
Tibial Hemimelia (TH) infected calves are born with twisted legs and fused joints, and/or abdominal hernias and misshapen skulls. Many are born dead, but if they are born alive are euthanized because they cannot stand or nurse. This syndrome was
first described in 1951 in Scotland in Galloway cattle, and was later described in USA Galloway calves in 1974. Later TH variants were seen in Shorthorns in 1999 (and SH based club calves). The Shorthorn TH mutation was traced to the Irish import bull Deerpark Improver #3684142 (1972), a rare direct Shorthorn import to North America. Improver was used extensively in the U.S. in the 1970s. Another variant traces to TKA Outcast (2001) a club calf sire. (Shorthorn)
Weak Calf Syndrome (IARS); or Isoleucyl-tRNA synthetase (a protein coding gene) disorder. IARS disorder causes early embryonic death & abortion. Calves that survive arrive with low birth weight after normal gestation, with weakness and poor suckling. IARS is found in Japanese and Australian Wagyu.
Weaver Disease Bovine Progressive Degenerative Myeloencephalopathy is a Brown Swiss defect, given its common name due to the weaving gait of affected animals. This defect attacks the brain and spinal cord. Symptoms include lack of coordination, slight swaying when standing, hind feet close together or crossing, leaning, and loss of control. Symptoms are generally noticeable between 6-18 months of age. The animal usually doesn’t die until 2-3 years of age, but will eventually suffer from malnutrition and muscle atrophy in addition to the original symptoms. The Brown Swiss Association used to recommend keeping the animal off concrete or other hard surfaces to prevent injury, and to have a veterinarian euthanize the animal when it can no longer eat or drink. (Brown Swiss)
![]()
RESOURCES:
AgriGenomics Mansfield, Illinois
Biogenetic Services, Brookings, South Dakota
Neogen, GeneSeek®, Igenity, SeekSire, Neogen Genomics®, Lansing Michigan
Genetic Visions, Middleton, Wisconsin
MMI Genomics, Davis, California
National Beef Cattle Evaluation Consortium
Pfizer Animal Genetics, Harahan, Louisiana
Reprotec, Tucson, Arizona
UC Davis Veterinary Genetics Lab (VGL), Davis, California
Zoetis Genetics (formerly Pfizer Animal Health), Kalamazoo, Michigan
List of Genetic Defects in the British Blue Cattle of UK, and DNA test status
Angus.org
ghr.nlm.hih.gov
ShorthornCountry.net
Beef Shorthorn Society
The American Grey Steppe Cattle Association, Borntograze.com
Veterinary Medicine (Eleventh Edition), 2017
Color Patterns in Crossbred Beef Cattle 2019 .pdf
Color Patterns in Crossbred Beef Cattle 2017 Oklahoma State University
Baby Calf Health Drovers Journal, 2011, (Dr. Tony Knight, CSU)
Genetic Diseases of Cattle (researchgate.net 2010)
Heritable Congenital Defects in Cattle VeterianKey.com
Genetic Defects, Beef Cattle, April 20, 2015, by Alison Van Eenennaam, University of California, Davis
Genetic defects are hereditary conditions that result in an undesirable phenotype (i.e. disease or trait). Genetic tests now exist for many of these genetic conditions. This fact sheet details the different conditions that have been identified in breeds and the companies that do genetic testing in cattle. This article and pdf document provides an excellent overview of the genetics of cattle (and all animals), and lists tests available for different breeds of cattle and the labs that provide them.
Genetic Defects • www.eBEEF.org • 2014-9 printable pdf version.
Congenital & Inherited Anomalies of the Musculoskeletal System Merck Veterninary Manual .pdf
Related Article: From Big to Small to Big to Small: A 3-part Pictorial History of Cattle Type Changes Over the Years, by Harlan Ritchie. This illustrates why DNA from one breed can sometimes be found in other breeds. Some trends happened "too quickly" in history.
Struck A-K, Dierks C, Braun M, Hellige M, Wagner A, Oelmaier B, Beineke A, Metzger J and Distl O. (2018)
A recessive lethal chondrodysplasia in a miniature zebu family results from an insertion affecting the chondroitin sulfate domain of aggrecan. BMC Genetics 19:91.
Policies Regarding Undesirable Genetic Factors, American Jersey Cattle Association, November 2, 2018.
Wagyu International, Inherited Recessive Genetic Testing for U.S. Red and Black Wagyu Cattle A Fact Sheet and Guide for Producers – revised 11/01/2014
HCA list of North American Veterinary Genetic Lab that offer DNA tests for cattle
article » How To Get Started With DNA Testing pdf, beef-cattle.extension.org
The genetic ancestry of American Creole cattle inferred from uniparental and autosomal genetic markers. Cattle imported from the Iberian Peninsula spread throughout America in the early years of discovery and colonization to originate Creole breeds, which adapted to a wide diversity of environments and later received influences from other origins, including Zebu cattle in more recent years. Published online: 07 August 2019.
Genetics of Coat Color in Cattle. Want to understand the genetics behind breeding cattle a bit better? This is an online reference book written to help ranchers and agriculture students understand genetics relevant to beef cattle. Inheritance Patterns; Inheritance; Mendelian Inheritance; Chromosome Considerations in Cattle; Fertility; Cattle Traits of Interest; Polled, Horned, and Scurred; Coat Color; Behavior Genetics; Genetics of Beef Quality; DNA Testing for Cattle; Gene Mapping; General References and Links to Cattle Genetics Sites. By Sheila M. Schmutz, Ph.D., Department of Animal Science, University of Saskatchewan, Canada.
DNA: Farmers Articles Increases in the use of artificial insemination, and in turn the narrowing of the bovine gene pool in recent decades has led to the surfacing and accumulation of genetic defects. Many producers are not fully aware of educated about these defects until one of them hits their herd. By knowing how these defects can affect your herd, and where they originate, you can reduce their occurrence to protect your bottom line.
From Two Bulls, 9 Million Dairy Cows... Just two Y chromosomes exist within a population of 9 million Holsteins. When researchers at the Pennsylvania State University looked closely at the male Holstein lines a few years ago, they discovered more than 99 percent of them can be traced back to one of two bulls, both born in the 1960s. That means among all the male Holsteins in the country, there are just two Y chromosomes. What traits have been lost over time?
A cattle graph genome incorporating global breed diversity, Feb 17, 2022. (nature.com ›› articles ›› s41467-022-28605-0) Generating African genome assemblies. Global cattle breeds display high levels of genetic diversity. Despite only 8% of cattle being found in Europe, European breeds dominate current genetic resources, and represent only a small fraction of bovine diversity. Cattle are one of the most populous farmed animals worldwide, with their global population of almost one billion, second only to chickens. Due to their use as draft animals and their ability to convert low-quality forage into energy-dense muscle and milk, they provide a significant source of nutrition and livelihood to over 6 billion people. Since their domestication almost 10,000 years ago, hundreds of distinct cattle breeds have been established, displaying a diverse range of heritable phenotypes, from differences in production phenotypes such as milk yield, to environmental adaptation, disease tolerance and altered physical characteristics such as horn shape and skin pigmentation. This phenotypic diversity between cattle breeds is mirrored by substantial genetic diversity, but this is poorly reflected by current reference resources. The primary reference genome is derived from a single European Hereford cow and projects such as the 1000 bulls genomes project are heavily skewed towards European-derived breeds (Bos taurus taurus) due to a number of factors such as geographic distribution and sample accessibility. Although European breeds largely all originate from the same domestication event that occurred in the Middle East, at least one further domestication event occurred in South Asia giving rise to the humped indicine breeds (Bos taurus indicus). These two Bos lineages have been estimated to have last had a common ancestor over 210,000 years ago, meaning the current Hereford reference genome particularly poorly represents the indicus sub-species.
![]()
 |
Homestead & Miniature Cattle Directory Homestead Cattle Association Mini Cattle Registry Bucking V Outfit, LLC. enterprises, est. 1990 Maricopa County, Arizona |
![]()
published online March ©2018 by Vintage Press, LLC.
updated October 2022